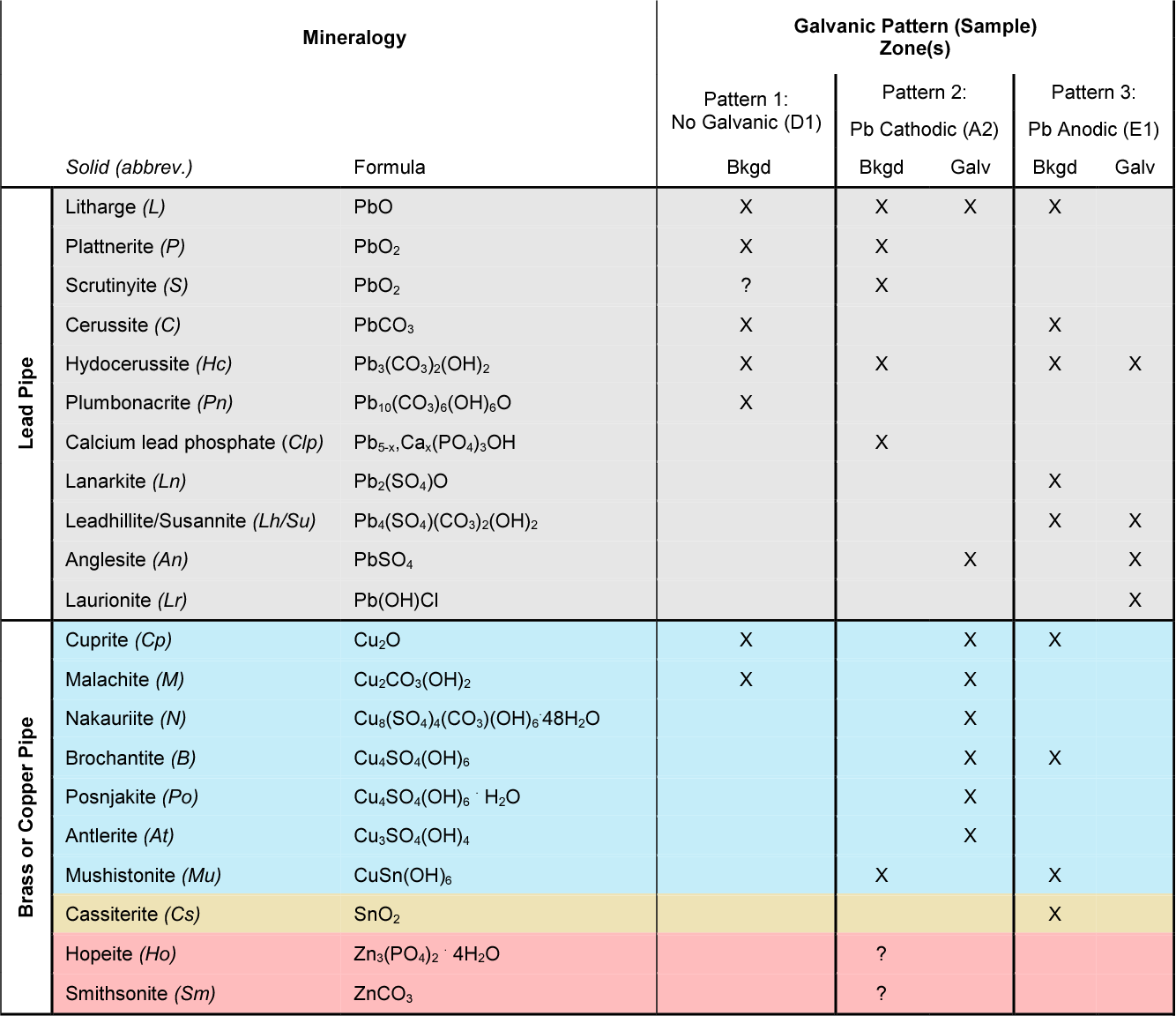
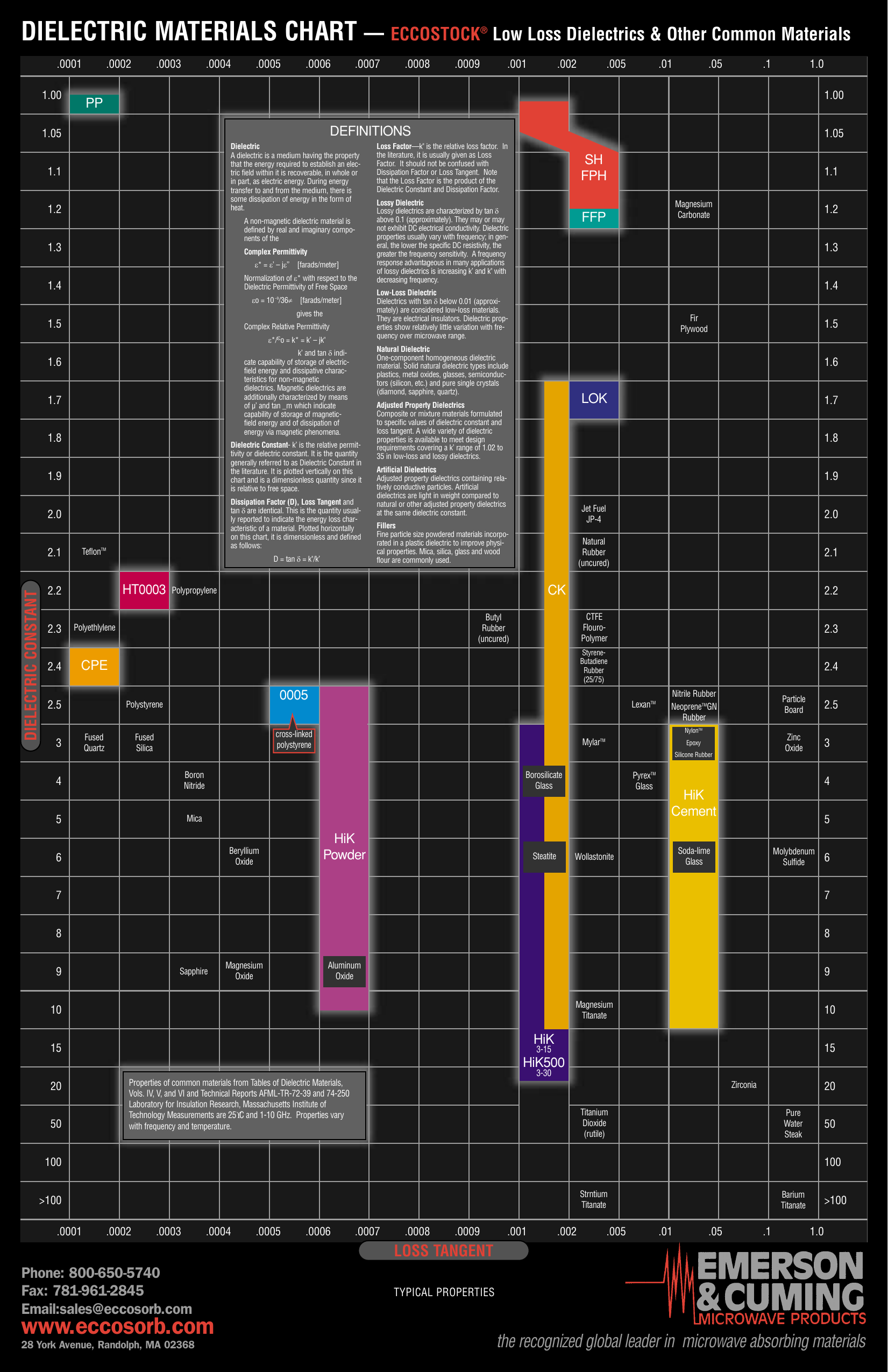
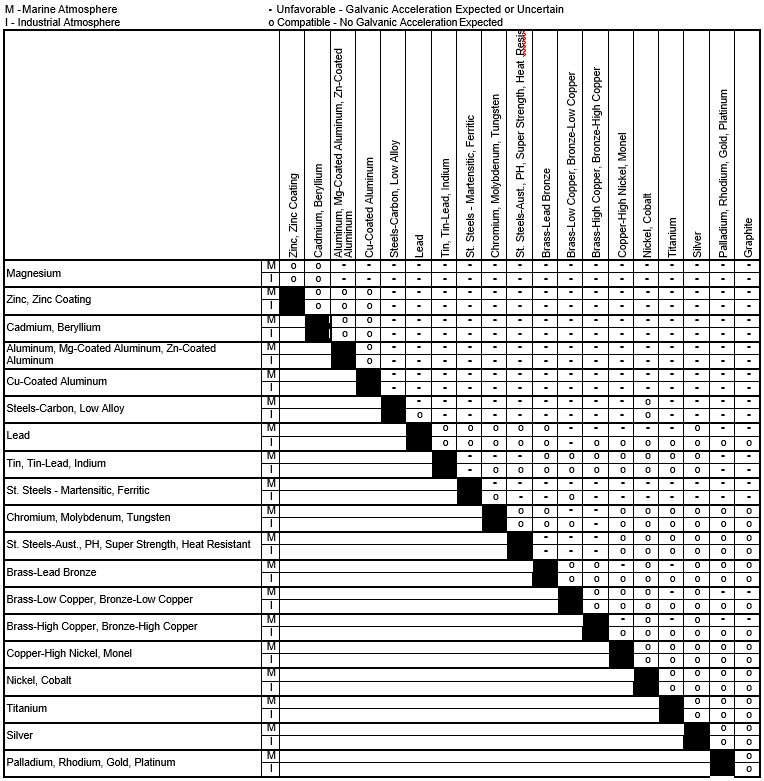
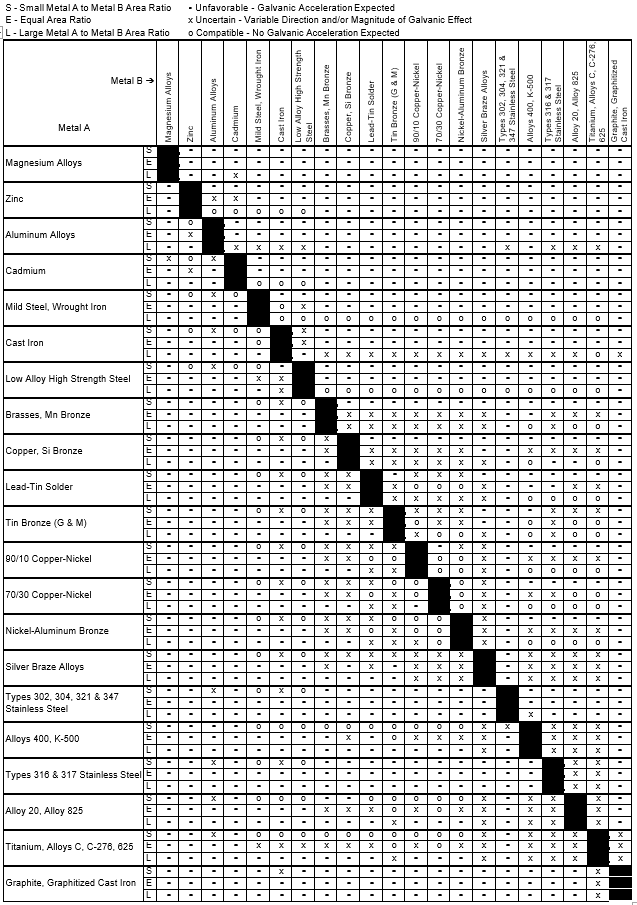
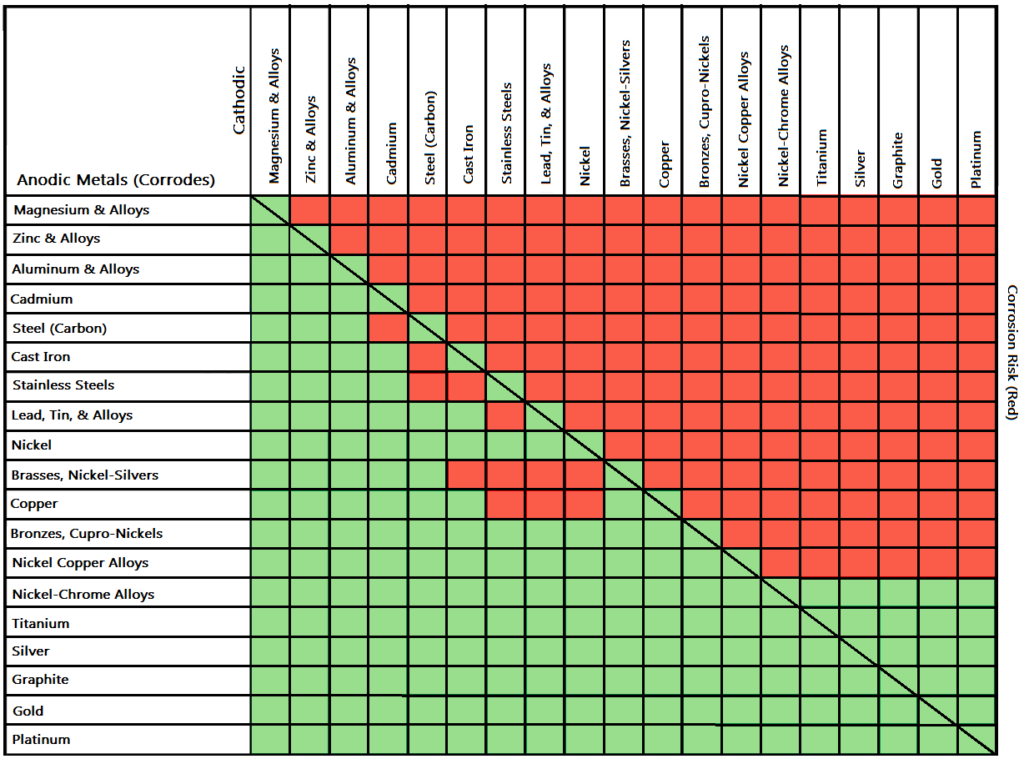
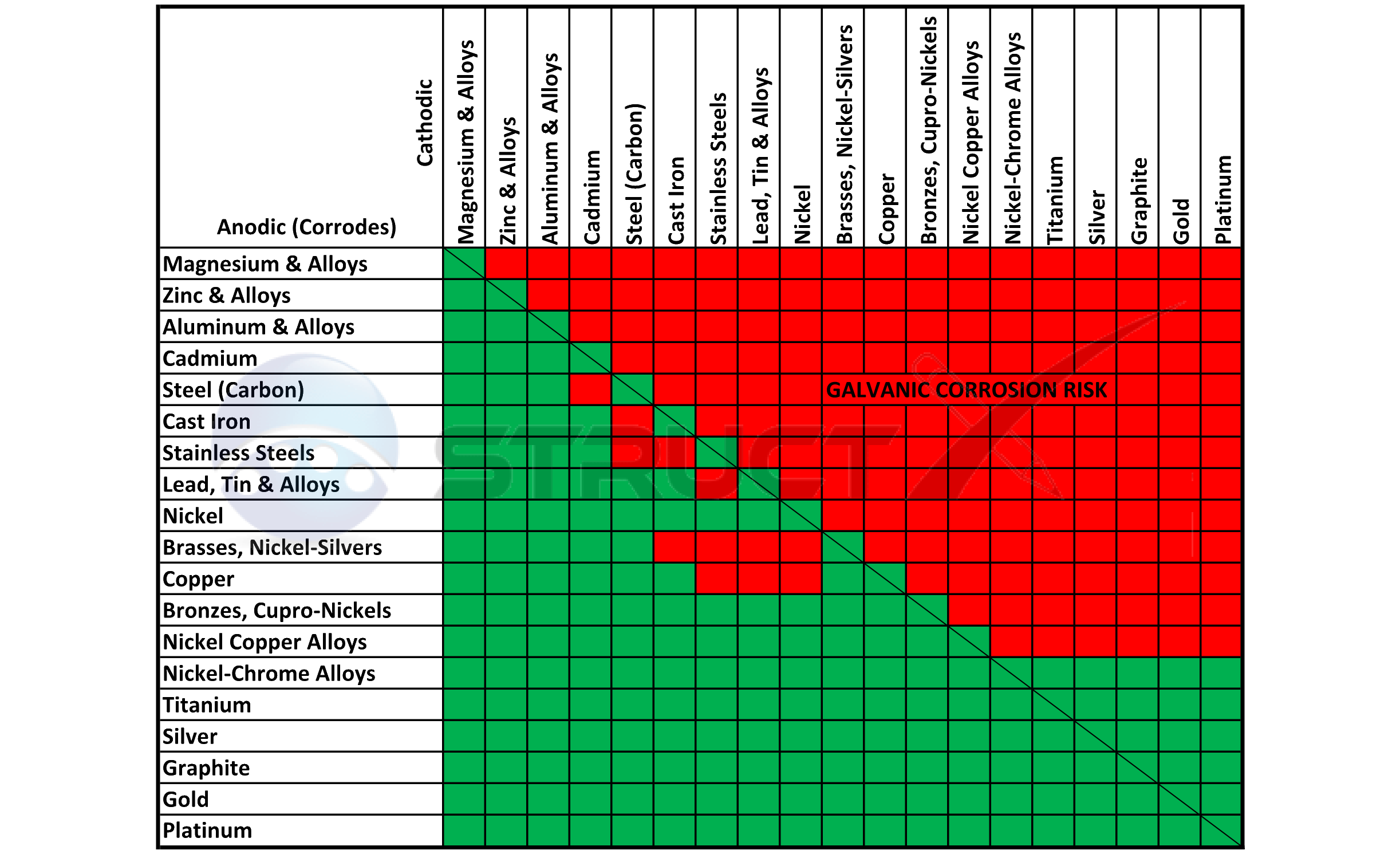
Dielectric Corrosion Chart
Dielectric Corrosion Chart - Web there are three conditions that must exist for galvanic corrosion to occur. The most active metals in the galvanic corrosion chart, like aluminum, zinc,. Web galvanic corrosion occurs when two different metals or alloys with different nobilities and therefore different electrochemical potentials come into contact with each. Web there are two primary types of galvanic cells that cause corrosion: Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web find out how different metals will corrode when placed together in an assembly based on their galvanic corrosion potential. When dissimilar metals are used together in the presence of an electrolyte,. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. It includes a chart that shows how different plating materials react to one another with. Second, there must be an. Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. Web the galvanic corrosion table ranks metals from the most “active” to the least active. This phenomenon is named after italian ph… First there must be two electrochemically dissimilar metals present. By eliminating any one of. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. See the chart with anodic, cathodic, and neutral. First there must be two electrochemically dissimilar metals present. Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised. Web there are two primary types of galvanic cells that cause corrosion: Web this article examines how dissimilar metals can lead to galvanic corrosion. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). This phenomenon is named after italian ph… The most active metals in the. Web there are three conditions that must exist for galvanic corrosion to occur. Web galvanic corrosion occurs when two different metals or alloys with different nobilities and therefore different electrochemical potentials come into contact with each. Web find out how different metals will corrode when placed together in an assembly based on their galvanic corrosion potential. The alloys near the. By eliminating any one of. First there must be two electrochemically dissimilar metals present. Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is. Web the following table was developed by interpreting available corrosion data and indicates the impact of electrical potential, environment, and surface area ratios to predict the. The corroded area was machined out and rebuilt with alloy 625 filler metal which is. Web find out how different metals will corrode when placed together in an assembly based on their galvanic corrosion. Web there are two primary types of galvanic cells that cause corrosion: Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. Web the galvanic corrosion table ranks metals from the most “active” to the least active. Web galvanic corrosion is the damage of. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). By eliminating any one of. This phenomenon is named after italian ph… Web the galvanic corrosion table ranks metals from the most “active” to the least active. The most active metals in the galvanic corrosion chart, like. Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web the galvanic corrosion table ranks metals from the most “active” to the. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web there are two primary types of galvanic cells that cause corrosion: Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. Web find out how different metals will corrode when placed together in an assembly based on their galvanic corrosion potential. For any. The corroded area was machined out and rebuilt with alloy 625 filler metal which is. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Second, there must be an. The alloys near the bottom are cathodic and. Web there are two primary types of galvanic cells that cause. Web galvanic corrosion occurs when two different metals or alloys with different nobilities and therefore different electrochemical potentials come into contact with each. Web this article examines how dissimilar metals can lead to galvanic corrosion. See the chart with anodic, cathodic, and neutral. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web the “galvanic series of metals and alloys” chart above provides a realistic and practical ranking of metallic electrical potentials. Web there are two primary types of galvanic cells that cause corrosion: For any combination of dissimilar metals, the metal with the lower number will act. Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. This phenomenon is named after italian ph… Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. By eliminating any one of. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. The alloys near the bottom are cathodic and. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. The corroded area was machined out and rebuilt with alloy 625 filler metal which is.Galvanic Action Corrosion Prevention Architect's Blog
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Dielectric Chart
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Corrosion charts Graphite Technology
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Galvanic Corrosion Common Questions Answered
Dissimilar Corrosion Materials Tables
Galvanic Series (electrochemical series)
Web Metals Listed On The Top Of The Chart (Anodic) Will Corrode Faster Than The Metals On The Bottom Of The Chart (Cathodic).
Web There Are Three Conditions That Must Exist For Galvanic Corrosion To Occur.
The Most Active Metals In The Galvanic Corrosion Chart, Like Aluminum, Zinc,.
Web Galvanic Corrosion, Also Known As Bimetallic Corrosion Or Dissimilar Metal Corrosion, Is An Electrochemical Process That Occurs When Two Different Metals Are In.
Related Post: